Abstract
Haploidentical donor hematopoietic cell transplantation (HaploHCT) using high-dose post-transplant cyclophosphamide (PTCy) has been increasingly used in patients with hematologic disorders with promising results. However, limited data is available on the incidence, pattern, and risk factors for cytomegalovirus (CMV) reactivation after HaploHCT with PTCy. Furthermore, the impact of CMV reactivation on HaploHCT outcomes has not been well understood in these patients. In this retrospective study we evaluated 119 consecutive patients undergoing HaploHCT for hematological diseases using PTCy between January 2008 and December 2016 at City of Hope under the IRB approved protocol. CMV reactivation was monitored by our institutional PCR assay (quantitative detection limit: 500gc/ml, qualitative limit: 250gc/ml) at least once a week for 100 days post-HCT, with preemptive anti-CMV therapy for positive PCR according to the institutional guidelines.
The median age of the cohort was 43 years (range: 2 to 71 years); 47 patients were female and 72 were male. CMV serostatus of donor/recipient was D-R- in 7, D+R- in 6, D-R+ in 23, and D+R+ in 82 patients. Patients received fully ablative (n=46) or reduced-intensity/non-myeloablative conditioning (n=73) followed by peripheral blood stem cell (n=81) or bone marrow (n=38) graft from sibling (n=42) or non-sibling Haplo donor (n=77). Graft versus host disease (GVHD) prophylaxis was PTCy plus tacrolimus/mycophenolate mofetil. Diagnoses of these patients were acute leukemia (n=80), bone marrow failure (n=15), lymphoma (n=11), chronic leukemia (n=6), hemoglobinopathies (n=5), and multiple myeloma (n=2). The HCT-comorbidity index was more than 2 in 42% (n=50) of the patients.
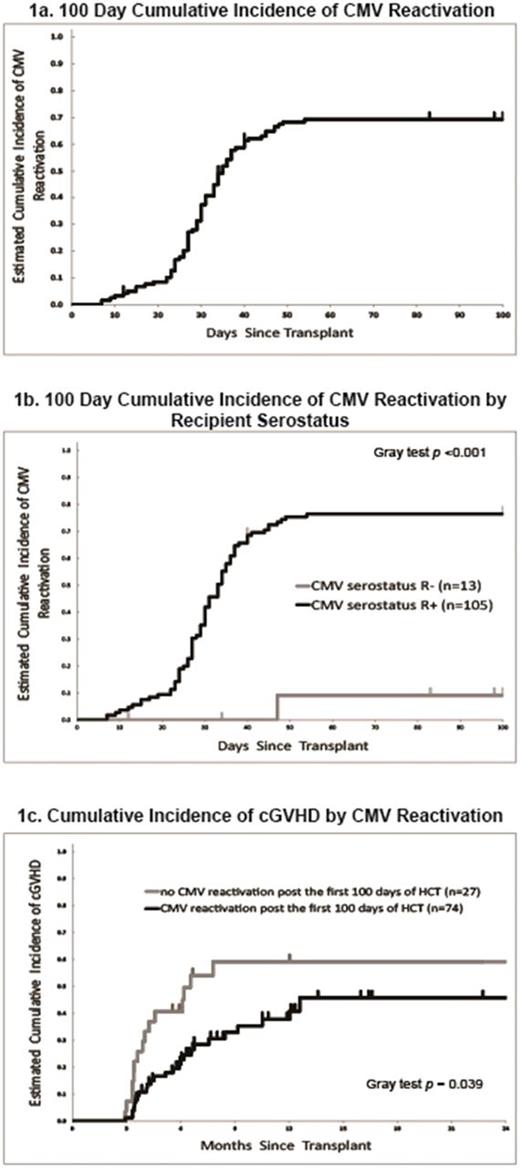
Cumulative incidence (CI) of CMV reactivation for the entire cohort was 69.2% (95%CI: 59.8-76.8%) at 100-days, with the median time to reactivation at 35 days (Figure 1a). While for seropositive recipients (n=105), cumulative incidence of CMV reactivation was 76.5% (95%CI: 66.9-83.6%) with the median onset at 33 days. The median CMV PCR values at onset was 624 gc/ml (range: <500 - 9,930) while the median peak PCR value during the 100-day period was 2965 gc/ml (range: <500 - 221,125). The median duration of CMV reactivation was 21 days and 28% (n=33) of patients had recurrent CMV reactivation during the first 100 days post-HCT. In our cohort there was no patient who developed CMV disease.
In univariate analyses the only significant risk factor for CMV reactivation was recipient serostatus (HR=0.06, 95%CI: 0.01 to 0.40 for R- compared to R+, p<0.001) (Figure 1b). Acute GVHD (aGVHD) was not associated with CMV reactivation when analyzed as a time-dependent variable partly due to lower incidence and later onset of aGVHD (100 days incidence: 36.7%; median onset day was not reached, range= 18 to 88) than CMV reactivations (median: 33 days, range= 7 to 54).
We then evaluated the impact of CMV reactivation in a landmark analysis of 101 patients who were relapse free for 100 days. The median follow up duration in this subgroup was 12.3 months (range: 3.4 to 99.1). In univariate analysis, CMV reactivation was not associated with overall survival (OS), relapse-free survival (RFS), relapse incidence, or non-relapse mortality (NRM). Similarly, the high-peak CMV reactivation or prolonged CMV reactivation (total duration or total episodes) was not predictive of poor outcomes in this cohort. Interestingly, the risk of chronic GVHD was greater in patients who had no CMV reactivation than those who developed prolonged/recurrent CMV reactivations (56.0% vs. 20.5%, p= 0.048, Figure 2).
In conclusion, HaploHCT with PTCy was associated with a high rate of CMV reactivations in seropositive recipients. However, due to pre-emptive therapy approach, CMV reactivation did not translate into higher rate of CMV disease. Despite many patients requiring a long course of preemptive anti-CMV therapy, there was no obvious association between CMV reactivation and survival outcomes. The observed association between CMV reactivation and reduced risk of chronic GVHD warrant further clinical and correlative immunologic studies.
Nademanee: seattle Genetics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal